Abstract
Background: Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is a promising target for cancer treatment as its expression enhances tumor cell growth and survival, promotes epithelial-mesenchymal transition and metastasis and participates in pro-survival pathways such as PI3-Kinase/AKT, EGFR signaling, and the p38-MAPkinase pathway. ROR1 is undetectable in normal adult tissues, however aberrant expression of ROR1 in malignancies has been detected in B-cell malignancies such as B-cell acute lymphoblastic leukemia (B-ALL), diffuse large cell B-cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL), and mantle cell lymphoma (MCL). Furthermore, upregulated expression has been detected in various solid tumors, including ovarian cancer, breast adenocarcinomas encompassing triple negative breast cancer (TNBC), pancreatic cancer, Ewing's sarcoma and lung adenocarcinoma. The increased expression of ROR1 in hematological and solid tumor malignancies has been associated with tumor proliferation, metastasis and poor clinical outcomes. The autologous UltraCAR-T® platform is designed to overcome the limitations of traditional CAR T which leads to an exhausted T cell phenotype, high manufacturing costs, and treatment delays, using advanced non-viral gene delivery system and a decentralized, overnight manufacturing process.
Scientific Rationale: PRGN-3007 is built on Precigen's UltraCAR-T platform and is engineered to simultaneously express a ROR1-specific chimeric antigen receptor (ROR1 CAR); membrane bound interleukin-15 (mbIL15) for enhanced in vivo expansion and persistence; a kill switch for improved safety profile; and a novel mechanism for the intrinsic blockade of programmed cell death protein 1 (PD-1) gene expression. Preclinical studies with PRGN-3007 UltraCAR-T cells showed significant reduction in PD-1 expression with increased ROR1 specific cytotoxicity and inflammatory cytokine production upon co-culture with ROR1+ PD-L1+ hematological and solid tumor cells. Furthermore, PRGN-3007 was selectively and effectively eliminated by the kill switch activator antibody treatment. In vivo, a single administration of PRGN-3007, effectively reduced tumor burden and significantly improved overall survival (p<0.05) of tumor bearing mice compared to Control ROR1 CAR-T in an aggressive xenograft model of mantle cell lymphoma. Blood analyses demonstrated sustained downregulation of PD-1 expression, rapid expansion, long-term persistence, and a predominant central memory phenotype of PRGN-3007 in tumor bearing mice. (Blood (2021) 138 (Supplement 1): 1694.)
Study Design: This is a first-in-human, Phase 1/1b dose escalation/dose expansion study to evaluate the safety and effectiveness of PRGN-3007 UltraCAR-T cells in patients with advanced ROR1-postive (ROR1+) hematologic (CLL, MCL, ALL, and DLBCL; Arm 1) and solid tumors (TNBC; Arm 2). Key inclusion criteria include absolute lymphocyte count ≥ 0.2k/µL, KPS > 70, adequate organ function, confirmed expression of ROR1 and: relapsed or refractory CLL; pathologically confirmed ALL B-cell precursor and measurable disease, pathologically confirmed MCL with overexpression of cyclin D1 or presence of t (11;14); pathologically confirmed DLBCL or high grade B-cell lymphoma including transformation from low grade lymphoma; or locally advanced unresectable or metastatic histologically confirmed TNBC with measurable disease as per RECIST 1.1.
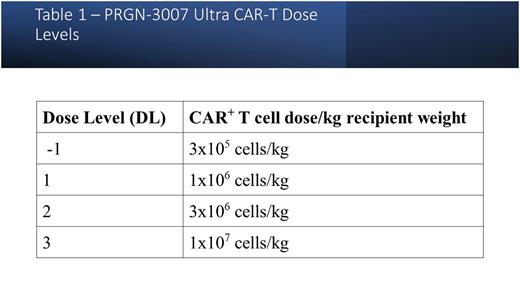
Study subjects undergo leukapheresis followed by lymphodepletion with either fludarabine 30mg/m2 and cyclophosphamide 500mg/m2 for 3 days (Arm 1) or 60 mg/kg cyclophosphamide for 2 days (Arm 2). The study will enroll in 2 phases: an initial dose escalation phase followed by a dose expansion phase. Up to 4 dose levels are planned in dose escalation phase (Table 1), and the MTD will be determined separately in subjects in the hematologic arm and the solid tumor arm. The MTD for each arm will be expanded independently in the dose expansion phase of the study. All subjects will be followed for adverse events, CAR-T-related toxicities, disease response and PRGN-3007 cell expansion and persistence. In addition, the mechanisms of safety and effectiveness of PRGN-3007 cells will be evaluated with correlative assays of specific immune response pathways.
Disclosures
Pinilla Ibarz:AstraZeneca: Consultancy; SecuraBio: Research Funding; AbbVie: Consultancy; Pharmacyclics: Consultancy; Janssen Pharmaceuticals: Consultancy. Lankford:Precigen: Current Employment, Current equity holder in publicly-traded company. Sabzevari:Precigen: Current Employment, Current equity holder in publicly-traded company; Kinnate Biopharma: Membership on an entity's Board of Directors or advisory committees. Shah:Servier: Other: grants and investigator-initiated trials; PeproMene Bio: Other: Steering committee; Autolus: Consultancy; Century Therapeutics: Consultancy; Adaptive: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy; Acrotech: Consultancy; Jazz: Consultancy, Other: grants and investigator-initiated trials; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy, Other: grants and investigator-initiated trials; BMS/Celgene/Juno: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Amgen: Consultancy. Chavez:ADC Therapeutics: Research Funding; Abbvie: Consultancy; Merck: Research Funding; MorphoSys/Incyte: Speakers Bureau; Janssen: Research Funding; TG Therapeutics: Honoraria; GenMab: Consultancy; Astrazeneca: Research Funding, Speakers Bureau; Beigene: Honoraria; Adicet: Consultancy; Epizyme: Honoraria, Speakers Bureau; Kite Pharma: Consultancy. Shah:Precigen: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal